Density of Water
 The density of water changes at different temperatures. The graph shows how
The density of water changes at different temperatures. The graph shows how
this change is not obvious. In fact, water is most dense at 4 oC.
You can download large tables of data to find the density at specific temperatures (or vice versa).
It is much easier to use this online calculator (don't forget to cite the source)
Online Density of Water Calculator
Boiling Point of Water
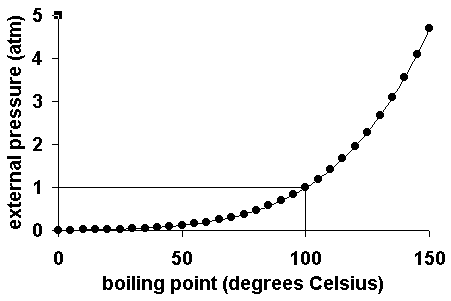 The boiling point of water depends on the atmospheric pressure above the surface of the water. As pressure increases, higher temperatures are required.
The boiling point of water depends on the atmospheric pressure above the surface of the water. As pressure increases, higher temperatures are required.
In order to determine the accepted value for the boiling point of water, remember, the water must be pure (distilled) AND you need to measure the atmospheric (barometric) pressure using a barometer.
Once you know the pressure (usually measured in atmospheres or kPa) you can find the accepted value for pressure. You may need to convert (1 atm = 101.3 kPa)
Here is a detailed table outlining the changes in boiling point at different pressures.
In the lab, the table below would be more useful. You may need to convert the pressure measurement first.

Comments (0)
You don't have permission to comment on this page.